
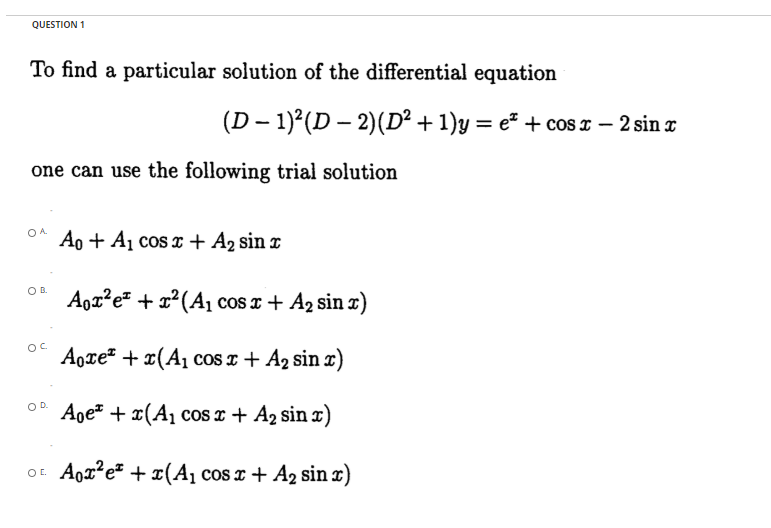
How do I log out of my account? Click on the profile icon in the upper right corner of your screen, and click Sign out in the dropdown. Help Center Click on the profile icon in the upper right corner of your screen, and click Sign out in the dropdown. How do you log out of all devices on Chegg? Herein, how many devices can be logged into a Chegg account?
CHEGG TRIAL SOLUTIONS CODE
When users violate our Honor Code policies, whether they are within the Chegg Tutor experience, while using Chegg Study, or across any of our products and services, we remove those users from the platform. 2 mobile apps on 2 different devices with the same eTextbook open should work fine.īeside above, is Chegg cheating? “ Chegg does not tolerate cheating. You can view your eTextbook on multiple devices at the same time if you are using the mobile app. Keeping this in consideration, can you use Chegg on multiple computers? Nope, as per my understanding, if you try to do so, your account will be made inactive or even they may revoke account for authentication issues. This instability negates the energy release that would happen from the breaking of the bond with the hydrogen, so it is less favorable to break.Are two simultaneous logins to Chegg accounts available? - Quora. Weak acids, however, form a very unstable ion when the hydrogen leaves. Because of this stability, it is favorable for the hydrogen to leave, since breaking that bond will release energy into the system. In the case of strong acids, the ion that is created when the hydrogen leaves is very stable. This stability means that the hydrogen is less likely to interact and bond with the water molecules and, therefore, more likely to remain with the acid. In the case of a weak acid, the molecule can participate in hydrogen bonding with water, making it much more stable in solution. Because the acid is not stable, the hydrogen is more likely to interact and bond with the water molecules instead of remaining with the acid. In the case of a strong acids, the molecule cannot form hydrogen bonds with water, making it unstable in solution.
Because of this difference in bond strength, the strong acid will be fully ionized, while the weak acid will be only partially ionized. For weak acids, the hydrogen is bound to the acid with a much stronger bond, making it harder for the water to pull it off. In the case of strong acids, the dissociation of the hydrogen is extremely favorable because the bond that will be formed between the hydrogen and water is much more stable than the bond that will break between the hydrogen and the acids. Because of this inaccessibility, the water cannot pull off many of the hydrogens, leading to incomplete ionization of the acid. We review their content and use your feedback to keep the quality high. Who are the experts Experts are tested by Chegg as specialists in their subject area. In the case of weak acids, the water has less access to the hydrogen (the hydrogen is less mobile and somewhat inaccessible to the water). Ask an expert Ask an expert done loading. Because of how accessible the hydrogen is to the water, the water can easily pull off the hydrogen, and will fully ionize the acid. In the case of strong acids, the hydrogens are very mobile and able to interact with the water easily. Because of this low solubility, many of the hydrogens do not dissociate in water, and remain attached to the acid. In the case of weak acids, the salts formed when the hydrogens dissociate are much less soluble.
CHEGG TRIAL SOLUTIONS FREE
Because of this high solubility, the hydrogens are free to dissociate away from the salt and interact with the water.

In the case of strong acids, the salt that the hydrogen is bound to is always very soluble in water (ex. Which of the following correctly describes what is happening on the molecular level for acids when dissolved in water? O A. Weak acids only partially dissociate, so the equilibrium must be considered. Transcribed image text: For calculating the pH of acid solutions, strong acids are treated as fully dissociating in water so equilibrium is ignored.


 0 kommentar(er)
0 kommentar(er)
